

They have the same number of protons in the nucleus as they have electrons orbiting in the energy levels around the nucleus. There are 2 protons in the helium nucleus. It does not store any personal data.If the number of neutrons N is 2 then there must be 4 - 2 = 2 protons. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The cookie is used to store the user consent for the cookies in the category "Performance". This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. The cookies is used to store the user consent for the cookies in the category "Necessary". The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously.

Necessary cookies are absolutely essential for the website to function properly. The neutrons and protons are contained within the nucleus, a tight central area of the atom. The number of neutrons can be calculated by subtracting the number of protons from the atomic mass, which is 16 minus eight for oxygen.An atom is primarily comprised of neutrons, electrons and protons. How do you calculate the number of protons and neutrons? Remember that the given atomic mass has to be rounded off to the nearest whole number before any further calculations. Subtract the atomic number from the atomic mass. The number of neutrons can be found simply by identifying the atomic mass (found at the bottom of the element on the periodic table) and the atomic number. Its atomic number correlates with the number of protons and electrons the element has, while the element’s neutrons can be determined from its mass. The gaseous element also has an atomic number of 2 and an atomic symbol of He. Helium has an atomic mass of 4.00 atomic mass units. The atomic number is specifically tied to a single element. This number clearly shows you how many protons an element’s nucleus contains.
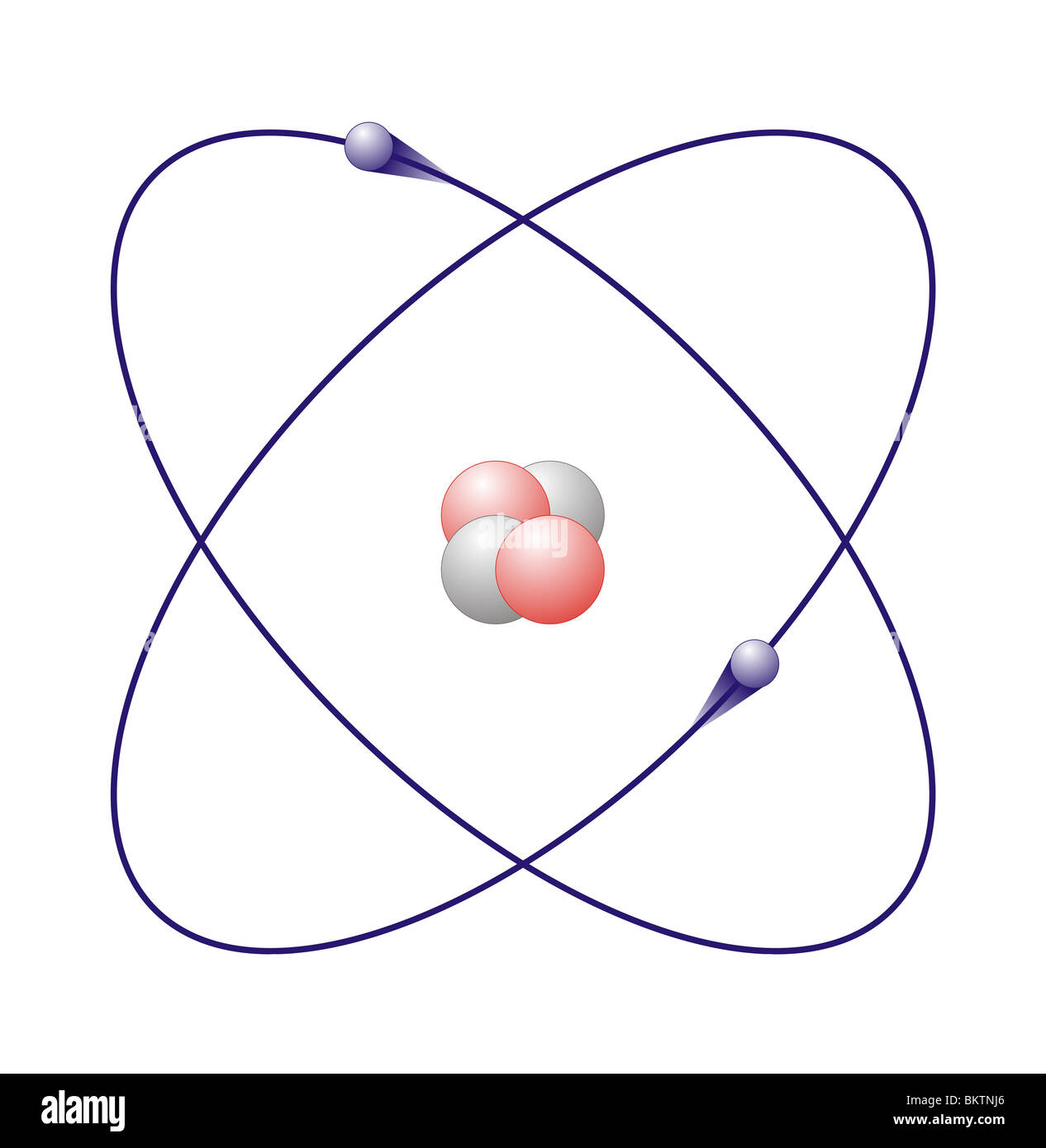
Helium can be found on the periodic table at atomic number two. Superfluid research stable isotopes of helium are helium-3 (or 3He), with two protons and one neutron, and helium-4 (or 4He), with two protons and two neutrons. He3 has two protons, one neutron, and two electrons. Helium can also have two unstable isotopes, helium-6 (four neutrons) and helium-8 (six neutrons). The most common, stable form of helium has two protons and two neutrons. The only exception is helium, which has just two electrons.
#HELIUM PROTONS NEUTRONS ELECTRONS FULL#
Their outer energy levels are full because they each have eight valence electrons. Noble gases are the least reactive of all known elements. Protons and one neutron, and helium-4 (or 4He), with two protons and two neutrons. Helium-4 is made of 2 protons (the part of an atomic nucleus with a positive charge), 2 neutrons (the part of an atomic nucleus with no charge) and 2 electrons (the part of an atom that goes around the nucleus with a negative charge). How many protons neutrons and electrons does helium 4 have?


 0 kommentar(er)
0 kommentar(er)
